You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Probing around teeth is a time-tested clinical method to determine the periodontal health of a tooth, detect ongoing inflammation, and evaluate the results of therapy.1 It allows the identification of sites with a history of periodontal disease or those at risk for periodontal breakdown. Periodontal probing is also used to locate calculus, measure gingival recession, detect furcation involvements, determine mucogingival relationships, and gauge bleeding tendencies.
Since the introduction of implant-supported restorations, probing methods similar to those used around teeth have also been used around dental implants in an effort to assess peri-implant tissue status. However, limitations in the information gleaned by probing along with differences in periodontal and peri-implant anatomy have raised questions regarding the value and risks of probing around implants.2
The purpose of this article is to discuss the risks and advantages of probing around teeth and dental implants and to suggest methods to allow more accurate evaluation of peri-implant conditions.
Rationale for Probing Around Natural Teeth
Clinical probing is the most commonly used parameter to document probing depth and assess loss of periodontal attachment around natural teeth.1 Probing depth that increases over time may indicate the need for additional diagnostic aids, such as radiography or bone sounding. However, there are two different probing depths: the biological/histological depth and the clinical probing depth.3 The biological depth is the distance between the gingival margin and the base of the pocket, which can only be measured using histological sections. Clinical probing depth is the distance to which a probe penetrates into the sulcus/pocket. The clinical probing pocket depth (PPD) is not congruent with the histological measurement, differing by an average of 0.3 mm to 0.5 mm.4 Nevertheless, clinical PPD is a non-invasive approach to attain information regarding clinical attachment loss and presence of inflammation in periodontal tissues.
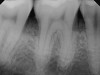
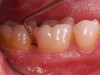
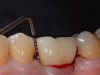
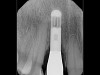
Clinical PPD is measured to the nearest millimeter by means of a graduated periodontal probe with a standardized tip diameter of approximately 0.4 mm to 0.5 mm. Several factors can influence the measurements made with periodontal probes, including: the thickness of the probe used5; the angulation and positioning of the probe depending on anatomic features, such as the contour of the tooth surface (Figure 1 through Figure 3)6; the graduation scale of the probe5; the pressure applied on the instrument during probing6; and the degree of inflammatory cell infiltration in the soft tissue and accompanying loss of collagen.7
A decrease in PPD after treatment indicates an improvement in the health of the periodontal tissues. However, a change in PPD alone following periodontal treatment does not necessarily indicate the formation of a new connective tissue attachment at the site of the previous lesion, but rather reflects a resolution of the inflammatory process, which may reoccur without an accompanying histologic gain of attachment.7 Therefore, a more accurate assessment of periodontal health uses both PPD and gingival marginal recession at the site of probing. Subtracting the latter from the former is a measure of the clinical attachment level, which determines the actual loss of soft-tissue attachment. This requires a fixed reference point, usually the cementoenamel junction (CEJ), to standardize the measurement from the gingival margin. This measurement, together with radiographic bone loss or sounding of marginal bone levels, is an indicator of the extent of tissue loss. This information can help determine the overall prognosis of the affected tooth as well as the success or failure of treatment.8
Bleeding on probing (BoP) is another indicator that can be determined through use of the periodontal probe for assessment of the periodontal tissue condition.9 A positive BoP indicates the gingiva is inflamed. Moreover, indices are available to quantify (albeit subject to probing limits) this parameter.10,11 As a single test, BoP is not a good predictor for attachment loss; however, the absence of bleeding indicates periodontal stability.12
Changes in probing depth measurement over time and BoP have been shown to be predicable clinical parameters to monitor periodontal health and ongoing diseases around natural teeth.13
Distinction Between Periodontal and Peri-implant Tissues
The soft-tissue barriers around dental implants differ from those around natural teeth.14 The gingival mucosa around teeth is covered by a keratinized oral epithelium, which is continuous with the junctional epithelium. The junctional epithelium terminates at the CEJ where acellular extrinsic fibers establish the supra-alveolar attachment apparatus. This is characterized by collagen fibers that project from the cementum into the connective tissue and the bone. These fibers include gingival, transseptal, and periodontal ligament fibers; all of these help anchor the soft tissue to the root of the tooth.
The soft-tissue barrier around implants consists of peri-implant mucosa covered by a keratinized oral epithelium, which is continuous with the junctional epithelium.15 Apical to the junctional epithelium, a zone of non-infiltrated, collagen-rich, but cell-poor connective tissue separates the apical part of the junctional epithelium from the alveolar bone. This zone, together with the junctional epithelium, establishes an implant–mucosa attachment that is approximately 3 mm to 4 mm in length. It has been demonstrated that the junctional epithelium of the peri-implant mucosa is attached to the implant surface via hemidesmosomes and basal lamina.16 Unlike the transcrestal fibers of natural teeth, the peri-implant collagen fibers run parallel to the implant surface.
Concerns for Probing Around Dental Implants
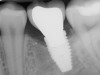
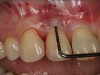
Similar to natural teeth, measurements of PPD and BoP before and after treatment are clinical parameters often used to determine the health of dental implants (Figure 4 and Figure 5). However, due to the difference between periodontal and peri-implant anatomy, concerns have been raised about the use of probes in peri-implant tissues around dental implants. These include the risk of peri-implant tissue damage,17,18 risk of damage to the implant surface by the metallic probe,19-21 risk of bacteria inoculation,22-27 and risk of galvanic corrosion resulting from contact between two dissimilar metals.21,28-30 These risks are discussed in the following sections.
Risk of Peri-implant Tissue Damage
The differences in soft-tissue composition between periodontal and peri-implant tissues and the lack of a fibrous attachment of the peri-implant mucosa to the implant surface have raised concerns regarding potential damage to the peri-implant tissue by probing. It is argued that the peri-implant mucosal seal may not have the ability to regenerate like natural teeth after a mechanical disruption of the epithelial attachment from clinical probing.17
However, Etter and colleagues evaluated the healing following standardized peri-implant probing using a force of 0.25 N and observed complete reformation of the mucosal seal after 5 days.18 They concluded that probing using a conventional periodontal probe with a light pressure of 0.25 N does not inflict irreversible damage to the soft-tissue seal in healthy peri-implant tissues and recommended the clinical probing as a reliable and reproducible diagnostic procedure to monitor the status of the peri-implant mucosa.18
Risk of Damage to the Implant Surface by the Metallic Probe
Implant probing involves inserting the probe between the supra-alveolar component of the implant complex (implant–abutment interface region) and the oral mucosal tissues surrounding it. The sides and tip of the probe may come in contact with the implant–abutment metal surfaces and rub against the metal collar at the apical third of the abutment. Some research has postulated that the rubbing of the probe against the implant–abutment metal surfaces may cause surface damage.19,20 A damaged implant–abutment metal surface can act as a nidus for bacterial accumulation and infection around the implant and may lead to implant failure.19,20 However, Fakhravar and colleagues investigated surface roughness on the apical collar of implant abutments caused by probing and scaling instruments and found that probing around implant abutments with a metal probe does not have an effect on abutment surfaces. In contrast, they found that instrumentation with scalers (metal and plastic) and plastic probes may cause surface roughness.21
Risk of Bacteria Inoculation
It has been speculated that clinical probing can possibly introduce bacteria into both periodontal soft tissues and the peri-implant mucosa. Bacteria could potentially be spread by inoculating the peri-implant soft-tissue seal. The supposition is that when a periodontal probe is contaminated at sites with high proportions of periodontal pathogens, the bacteria can attach to the probe and be transported from one site to another. Streptococcus mutans has been shown to be transmissible intraorally with a dental explorer.22 Light and electron microscopic observation showed that periodontal probes also have the potential to transmit pathogenic bacteria from involved sites to other sites in the mouth.23 Also, probes with indentations have been shown to harbor more pathogens than smooth probes.24
Christersson and colleagues transferred Actinobacillus actinomycetemcomitans from diseased pockets to healthy pockets with periodontal probes. The pathogens remained in the healthy pockets for 2 to 3 weeks; however, the inoculated pathogen did not lead to permanent colonization of the site.25 Holt and colleagues evaluated the potential for bacteria transmission as a function of the periodontal probe design. They showed that the total colony-forming unit was higher in deeper pockets regardless of types of probe design and suggested probing non-infected sites first before proceeding to probe sites with deeper pockets to minimize transmission.26
Despite the evidence of bacteria transmission through dental apparatus, Greenstein and Lamster in a critical review of the literature argued that the transfer of organisms does not necessarily result in colonization or infection of the host, which depends on a complex interaction between the host's susceptibility, number of virulent pathogens, local environment, and co-existing bacteria.27 Therefore, spread of bacteria and subsequent diseases on both implants and natural teeth from probing appears to be unwarranted.
Risk of Galvanic Corrosion
Most commercially available dental implants are made of pure titanium (CP-Ti) or titanium alloy Ti-6Al-4V.28 Titanium and its alloys have great resistance to corrosion in saline and acidic environments because of the stability of the titanium-oxide layer. When the stable oxide layer is broken down or removed and is unable to reform, titanium can be as corrosive as many other base metals.29
The hypothesis of a risk is that probing with a metal probe can lead to the removal of the oxide layer exposing the underlying titanium. In the presence of oral fluids, the contact between the metal probe and the dental implant, each of which is made of dissimilar alloys, can result in a flow of electric current between them due to the difference between their corrosion potential. The galvanic current causes acceleration of corrosion of the titanium and weakens the implant.30
To date, only one case of implant fracture due to galvanic current has been reported.31 The galvanic corrosion was attributed to the use of dissimilar metals between the dental implant and its superstructures. However, careful use of a smooth, rounded metallic probe has not been shown to cause galvanized corrosion.21
Rationale for Probing Around Dental Implants
Although the value of probing around dental implants has been questioned2 the consensus report of the sixth European workshop on periodontology recommended data collection on the following parameters for diagnosis of peri-implant diseases32: bleeding on probing, suppuration, probing depth, radiographic bone loss, implant mobility. Three out of these five parameters require the use of a periodontal probe for assessment.
Probing the sulcular depth around a dental implant is an important clinical exercise to assess implant health and stability. Longitudinal measurement of probing depth helps monitor the integrity of the peri-implant tissues. An increase in the probing depth usually indicates loss of alveolar bone support. Increasing probing depth over time in the presence of BoP and gingival exudate are criteria to diagnose peri-implant disease.33 Increased probing depth accompanied by BoP without concomitant bone loss has been defined as peri-implant mucositis.34 When these signs are present with radiographic (or sounding) evidence of bone loss, the disease has been defined as peri-implantitis.34
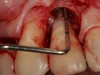
Probing is useful to determine the presence of biological complications at the buccal and lingual sites of implants, which cannot be evaluated on a radiograph (Figure 6 through Figure 8). Because of the absence of a periodontal ligament, bone loss on the buccal and lingual aspects of an implant indicates loss of support for the implant and may be a sign of additional circumferential bone loss around the implant.35
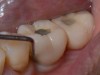
Due to the various aforementioned factors that can affect probing depth around an implant, a probing depth of 4 mm to 5 mm around an implant may not be as critical a diagnostic parameter as it would be around natural teeth (Figure 9 and Figure 10). However, probing depth accompanied by BoP becomes a significant concern around an implant. Therefore, while incidental measurement may not be indicative of the health status of the peri-implant tissue, probing at various time intervals enables assessment of health status and disease progression.
When assessing the periodontal health of a dental implant, understanding the differences between the soft tissue surrounding teeth and the soft tissue surrounding dental implants is essential. Dental implants have a weaker hemidesmosomal attachment.36 Therefore, probing using a very light force of 0.25 N is recommended. Excessive force on probing will lead to penetration of the cell-free zone leading to bone sounding instead of pocket measurement. This may not give an accurate assessment of the extent of the peri-implant disease.
Presence of bleeding on gentle probing is another useful parameter for diagnosis of peri-implant soft-tissue inflammation. In an experimental study Lang and colleagues showed healthy peri-implant sites had absence of BoP while increased BoP was seen at mucositis (67%) and peri-implantitis (91%) sites.37 The prognostic value of BoP was investigated in a prospective clinical study and this parameter was shown to be of high negative predictive values. In other words, the absence of bleeding can serve as an indicator for the presence of stable peri-implant conditions.38
Summary and Recommendations
In summary, risks of probing around natural teeth and dental implants include bacteria inoculation and spread of diseases. Improper probing can lead to undiagnosed diseases. Because the peri-implant attachment is weaker, bone sounding may result instead of probing. Probing around implants prior to osseointegration or around natural teeth prior to surgical healing can disturb and interfere with successful healing.
Today more implants are being placed to replace missing teeth.39 It has been reported that 45% of patients receiving implant therapy are likely to show signs of peri-implant diseases with varying degrees of severity throughout the lifespan of the implants.40,41 Clinical signs of the presence of peri-implant health or disease are critical. Similar to the benefits of probing around natural teeth, probing around implants remains the most efficient non-invasive method for clinical screening and assessment.
The following protocol is recommended for clinical probing around both natural teeth and dental implants: (1) use a smooth, unindented probe with a round tip diameter of approximately 0.4 mm to 0.5 mm; (2) use a gentle probing force of less than 0.25 N; (3) clean the probe in chlorhexidine after it is used in contaminated or infected sites (ie, in abscess areas around teeth or implants or in areas of moderate to advanced peri-implantitis with exudate) before using the instrument to probe other areas in the same patient.
To gain accurate data from probing around natural teeth or implants, the angle of the probe should be reproducible each time an area is probed. This may be difficult when teeth or implants are splinted or restored with pink or white composite materials designed to close interproximal spaces, or when a hybrid design has been used (Figure 11). In such cases, if the restoration is retrievable (either screw-retained or cemented with a temporary cement), it is advisable to remove the suprastructure before probing. If this cannot be done, taking a photograph of the position of the probe is helpful for probing at the same site at later intervals.
Additionally, accurate assessment of changes in soft- and hard-tissue loss can be achieved through the use of a fixed reference point, such as the CEJ, crown margin on natural teeth, or implant–abutment junction, or using a vacuum formed stent (Figure 12) on implants that can be notched in the areas where reproducible probing is desired.
Conclusion
Periodontal probing around natural teeth remains the most efficient and non-invasive method to diagnose loss of attachment, determine BoP (absence indicates health), monitor marginal recession (from a fixed reference point), and evaluate positive treatment outcomes (decrease in PPD). When used together with radiographs, PPD measurements can determine ongoing diseases/prognoses and the ability of clinicians and patients to manage biofilm and calculus.
Probing around dental implants is a non-invasive method to determine peri-implant diseases and whether radiographs are needed. Together with assessments of BoP, suppuration, and radiographs, probing can be used to determine the extent of peri-implant disease, suggest treatment methods (nonsurgical versus surgical management), and evaluate treatment outcomes. Probing around natural teeth and implants remains a valuable clinical parameter in assessing health, disease, and ongoing loss of soft and hard tissue around natural teeth and implants.
About the Authors
Stuart J. Froum, DDS
Adjunct Clinical Professor and Director of Clinical Research, Ashman Department of Periodontology and Implant Dentistry, New York University College of Dentistry, New York, New York; Private Practice, New York, New York
Wendy C.W. Wang, BDS, MSc
Consultant, Department of Dentistry, National University Hospital, Singapore
Queries to the author regarding this course may be submitted to
authorqueries@aegiscomm.com.
References
1. Takei HH, Carranza FA, Do JH. Clinical diagnosis. In: Newman MG, Takei HH, Klokkevold PR, Carraza FA. Carranza's Clinical Periodontology. 12th ed. St. Louis, MO: Elsevier Saunders; 2015:366-371.
2. Misch CE, Perel ML, Wang HL, et al. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17(1):5-15.
3. Armitage GC, Svanberc GK, Löe H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. J Clin Periodontol. 1977;4(3):173-190.
4. Listgarten MA. Periodontal probing: what does it mean? J Clin Periodontol. 1980;7(3):165-176.
5. Listgarten MA, Mao R, Robinson PJ. Periodontal probing and the relationship of the probe tip to periodontal tissues. J Periodontol. 1976;47(9):511-513.
6. Badersten A, Nilvéus R, Egelberg J. Reproducibility of probing attachment level measurements. J Clin Periodontol. 1984;11(7):475-485.
7. Van der Velden U, Abbas F, Winkel EG. Probing considerations in relation to susceptibility to periodontal breakdown. J Clin Periodontol. 1986;13(10):894-899.
8. McGuire MK, Nunn ME. Prognosis versus actual outcome. II. The effectiveness of clinical parameters in developing an accurate prognosis. J Periodontol. 1996;67(7):658-665.
9. Mühlemann HR, Son S. Gingival sulcus bleeding—a leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15(2):107-113.
10. Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533-551.
11. Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121-135.
12. Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990;17(10):714-721.
13. van der Velden U. Influence of periodontal health on probing depth and bleeding tendency. J Clin Periodontol. 1980;7(2):129-139.
14. Berglundh, T, Lindhe J, Ericsson L, et al. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2(2):81-90.
15. Schroeder HE, Listgarten MA. Fine structure of the developing epithelial attachment of human teeth. Monogr Dev Biol. 1971;2:1-134.
16. Gould TR, Brunette DM, Westsury L. The attachment mechanism of epithelial cells to titanium in vitro. J Periodontal Res. 1981;16(6):611-616.
17. Bauman GR, Mills M, Rapley JW, Hallmon WH. Clinical parameters of evaluation during implant maintenance. Int J Oral Maxillofac Implants. 1992;7(2):220-227.
18. Etter TH, Håkanson I, Lang NP, et al. Healing after standardized clinical probing of the peri-implant soft tissue seal: a histomorphometric study in dogs. Clin Oral Implants Res. 2002;13(6):571-580.
19. Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur J Oral Sci. 1998;106(1):527-551.
20. Esposito M, Hirsch JM, Lekholm U, Thomsen P. Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci. 1998;106(3):721-764.
21. Fakhravar B, Khocht A, Jefferies SR, Suzuki JB. Probing and scaling instrumentation on implant abutment surfaces: an in vitro study. Implant Dent. 2012;21(4):311-316.
22. Loesche WJ, Svanberg ML, Pape HR. Intraoral transmission of Streptococcus mutans by a dental explorer. J Dent Res. 1979;58(8):1765-1770.
23. Barnett ML, Baker RL, Olson JW. Material adherent to probes during a periodontal examination: light and electron microscopic observations. J Periodontol. 1982;53(7):446-448.
24. Papaioannou W, Bollen CM, Van Eldere J, Quirynen M. The adherence of periodontopathogens to periodontal probes. A possible factor in intra-oral transmission? J Periodontol. 1996;67(11):1164-1169.
25. Christersson LA, Slots J, Zambon JJ, Genco RJ. Transmission and colonization of Actinobacillus actinomycetemcomitans in localized juvenile periodontitis patients. J Periodontol. 1985;56(3):127-131.
26. Holt LA, Williams KB, Cobb CM, et al. Comparison of probes for microbial contamination following use in periodontal pockets of various depths. J Periodontol. 2004;75(3):353-359.
27. Greenstein G, Lamster I. Bacterial transmission in periodontal diseases: a critical review. J Periodontol. 1997;68(5):421-431.
28. Sykaras N, Iacopino AM, Marker VA, et al. Implant materials, designs, and surface topographies: their effect on osseointegration. A literature review. Int J Oral Maxillofac Implants. 2000;15(5):675-690.
29. Lemons JE, Lucas LC, Johansson BI. Intraoral corrosion resulting from coupling dental implants and restorative metallic systems. Implant Dent. 1992;1(2):107-112.
30. Speelman JA, Collaert B, Klinge B. Evaluation of different methods to clean titanium abutments. A scanning electron microscopic study. Clin Oral Implants Res. 1992;3(3):120-127.
31. Tagger Green N, Machtei EE, Horwitz J, Peled M. Fracture of dental implants: literature review and report of a case. Implant Dent. 2002;11(2):137-143.
32. Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):282-285.
33. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63-76.
34. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 suppl):286-291.
35. Fransson C, Wennström J, Berglundh T. Clinical characteristics at implants with a history of progressive bone loss. Clin Oral Implants Res. 2008;19(2):142-147.
36. Mombelli A, Mühle T, Bragger U, et al. Comparison of periodontal and peri-implant probing by depth-force pattern analysis. Clin Oral Implants Res. 1997;8(6):448-454.
37. Lang NP, Wetzel AC, Stich H, Caffesse RG. Histologic probe penetration in healthy and inflamed peri-implant tissues. Clin Oral Implants Res. 1994;5(4):191-201.
38. Jepsen S, Rühling A, Jepsen K, et al. Progressive peri-implantitis. Incidence and prediction of peri-implant attachment loss. Clin Oral Implants Res. 1996;7(2):133-142.
39. Dental Implants Facts and Figures. American Academy of Implant Dentistry website. http://www.aaid.com/about/press_room/dental_implants_faq.html. Accessed November 7, 2017.
40. Atieh MA, Alsabeeha NH, Faggion CM Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84(11):1586-1598.
41. Derks J, Schaller D, Håkansson J, et al. Effectiveness of implant therapy analyzed in a Swedish population prevalence of peri-implantitis. J Dent Res. 2016;95(1):43-49.