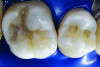
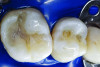
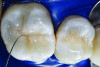
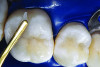
You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The longevity of the posterior composite restoration has been a subject of interest by many private practitioners, clinical studies, and research articles. The clinical performance of the original resin composite formulations was disappointing.1 These initial formulations were chemically cured, and their use was restricted to anterior cavity preparations. The filler particles were large, irregular, and unimodally distributed, and the filler content by weight was low.2
Early attempts to use composite resins in the posterior region revealed complications, including inadequate wear resistance,2-7 bulk fracture,3,7 a high polymerization shrinkage and lack of adaptation to the margins after polymerization, increased incidence of microleakage2,3,8 with frequent secondary caries and postoperative sensitivity,7,9-13 improper interproximal contact and contour,8 inadequate marginal adaptation,13 color instability,1,3 poor polish retention,1 pulpal irritation,5 and the potential need for endodontic therapy.1 Improving the clinical longevity of posterior resin composites in the oral cavity requires one to address these obstacles while developing an optimal protocol for placing posterior composite restorations.14,15
A successful restorative procedure for posterior resin composites relies on understanding and using a fundamental adhesive design concept involving biomaterial selection, adhesion, and clinical application. The fundamental principles of this process require maintaining sound tooth structure, ideally achieving a sterile, gap-free hybrid layer, and eliminating microleakage by securing a relatively stress-free restorative-tooth interface.16
Adhesive Design Concept
Unfortunately, many clinicians continue to perform outdated procedures with modern restorative materials, and then wonder why they continue to have microleakage, recurrent decay, and sensitivity. Advances in material science and adhesive technology require clinicians to modify their restorative techniques when placing adhesive restorations. This is particularly true when one is considering diagnosis, biomaterial selection, preparation design, restorative placement techniques, pulp protection, finishing, and maintenance.17 The adhesive design concept requires the selection of adhesive, bioactive restorative materials; simplified modifications of preparation designs; and precise placement procedures and techniques. This design concept has been instrumental in the paradigm shift from the principles of extension for prevention to the ultraconservative principle of prevention to eliminate extension. The proper interrelation of these restorative parameters—biomaterial selection, adhesion, and technique—can result in an optimal restorative–tooth interface with improved clinical performance (Figure 1), whereas an improper interrelation can lead to gap formation, microleakage, staining, sensitivity, caries, and partial or complete debonding of the restoration that can result in long-term clinical failure (Figure 2).18
Biomaterial Selection
Restorative material selection is a primary preoperative consideration of the adhesive design concept that should be completed in the diagnosis and treatment-planning phase prior to any final restorative treatment. When using composite restorations in the posterior region, the following clinical assessments should be considered:
• Anticipated dimensions and geometry of the preparation design and location of the margins19,20
• Tooth position in the arch19,21
• Location of proximal and occlusal contact sites
• Interrelationships with adjacent teeth and periodontal tissues22
• Size and the number of restorations23
• Structural defects (ie, incomplete fractures, erosion lesions, abrasion lesions)19,24
• Intra-arch and inter-arch protective functions
• Tooth anatomy and resistance
• Occlusion
• Esthetics
• Patients’ oral habits (ie, nail biting)
• Occlusal parafunctions (ie, bruxism and clenching)
• Ability to isolate the operative field19,23
In addition, consideration of particle size, distribution, and quantity represents crucial information in the determination of how to best use composite materials. Altering the filler component remains the most significant development in the evolution of resin composites.25 In general, mechanical and physical properties of composites improve in relationship to the amount of filler added. Many properties depend on the filler composition and type, including compression strength, hardness, flexural strength, tensile strength, elastic modulus, coefficient of thermal expansion, water absorption, and wear resistance.
Posterior restorations require highly-filled materials that provide sculptability, fracture resistance, color stability, hardness, and radiopacity, while conferring polishablity and the capacity to retain surface smoothness over time. Most of the current hybrid composites possess all of these characteristics. The specific preparation will determine the type of composite chosen for placement.
An important clinical consideration pertaining to polymerization shrinkage is the configuration factor (C-factor). The C-factor has a significant effect on the magnitude of shrinkage stresses that results from polymerization. It is the ratio of bonded to unbonded preparation surface areas on restorations26,27; the higher the C-factor, the greater the potential for internal stress and bond disruption. When considering direct placement of intracoronal restorations, preparations with a high C-factor have the greatest internal stress and should be restored with materials that develop lower contraction stress (low-shrinkage resin composite). Since the Class I cavity (Figure 3) has the highest C-factor (ie, Class I-5/1, Class II- 4/2) and greatest internal stress,28 this restoration is fundamental for developing adhesive research and improving our adhesive strategies.
Tooth Preparation Design
The adhesive application of newer formulations of resin composite materials permits a more conservative tooth preparation. This is based on the selected material having improved physical, mechanical, and optical characteristics similar to those of natural tooth structure.16,18 Therefore, it is not necessary to compensate for fracture resistance of the restoration by increasing the volume of restorative material at the restorative interface through tooth preparation.16 Furthermore, restoring the natural dentition with bonded composite reinforces the tooth and restoration, which results in an increased structural integrity while reducing and dissipating functional forces along the entire restorative interface.18 This adhesive process, bonding to enamel and dentin, provides unity, integrity, and strength to the restorative complex.29
General Considerations for Adhesive Preparation Design
Composite restorations use adhesive cavity preparation designs that require no geometric outline forms (Figure 4 and Figure 5).23,30 The modern adhesive preparation design employs a biologic approach that provides restoration retention through adhesion, in addition to reinforcement and strength to existing tooth structure. Although metal-free direct restorative systems depend upon the use of adhesive preparation designs that are more conservative, they also require more thorough adhesive techniques.23,30-33 For optimal success of posterior composite resin restorations, consideration should be given to tooth type (ie, molar, bicuspid) and location in the arch, as well as to the size and type of the carious lesion. Other considerations should include treatment of decayed or nondecayed unrestored teeth or restoration replacement. The clinician should also evaluate the relationships between occlusal function and preparation boundaries to place centric stops beyond or within the confines of the restoration. Final considerations should be made for the type of restorative technique (ie, direct or indirect), the quantity and quality of the remaining tooth structure and the mechanical forces exerted on it, the presence of defects, and the parameters for extension of the preparation to the esthetic zone.23,34
The following general guidelines should be followed for initial or replacement restorations for the posterior composite resin preparation:
-
Carious dentin can be removed using slow- and high-speed carbide burs and spoon excavators. The preparation is limited to access to the lesion or defect, since composites require less volume to resist clinical fracture than amalgam (Figure 6 and Figure 7).35 For Class II preparations, it is unnecessary to project the proximal extensions of the preparation into a contact-free zone.
-
The occlusal outline should eliminate all carious enamel, provide access to the carious dentin, eliminate any residual amalgam staining,18,33 and provide access for placing the restorative materials.33
-
The width of the preparation should be as narrow as possible, since the wear of the restoration is a direct function of that dimension.17,33 Additionally, the increased buccolingual width of the preparation can move the preparation margins into centric holding areas. For Class II preparations, in addition to minimizing the proximal box, the same is true for the depth of the gingival floor. In the case of resin composite, the depth should be established no further than the caries process. The greater the depth of the proximal box, the thinner the remaining gingival enamel margins to which the composite will bond.
-
Healthy tooth structures should only be removed when the occlusal outline requires extension beyond or within the previously indicated functional stops.33
-
The occlusal cavosurface margins of the preparation generally should not be beveled. Beveling automatically increases the width of the preparation, which in turn increases the potential for including the centric holding areas. Increased width also increases the potential for accelerated wear of the composite restoration.17,36 As the preparation is extended considerably toward the buccal and lingual surfaces, the inclusion of bevels along the occlusal margins may be appropriate. Beveling in this instance permits the bonded restoration to enhance the fracture resistance of the tooth.
-
To allow better resin adaptation, all internal line angles should be rounded and cavity walls should be smooth, as defined by the surface effects generated by a conventional round-ended preparation bur.37
Adhesion
Defined as the “molecular attraction exerted between the surfaces of bodies in contact,” adhesion occurs when unlike molecules are attracted.38 Conversely, cohesion occurs when molecules of the same kind are attracted. The adhesive, frequently a viscous fluid, comprises a material or film that joins two substrates together and solidifies. The adherend is the material or initial substrate to which the adhesive is applied.38
In dentistry, a surface sealant would be defined as a single adhesive “joint,” since only one interface exists. Adhesion or bonding is the process of forming an adhesive joint39; traditionally, two substrates are joined so that the adhesive produces two interfaces that are part of the adhesive joint. Although most adhesive joints involve only two interfaces, a bonded composite restoration would be an example of a more complex adhesive joint.39 The physical and chemical properties of the adhesive are the most important factors in the performance of adhesive joints, since these are the properties that maintain the integral bond. Ensuring adequate performance of the adhesive joint requires knowledge and experience in the types of adherend (ie, enamel, dentin, metal alloy, composite material) and the nature of the surface pretreatment or primer. The adhesive, adherend, and surface all impact the durability of the bonded structure.
The mechanical behavior of the bonded structure is influenced by the details of the joint design and by the way in which the applied loads are transferred from one adherend to the other. The specific energy of adhesion defined by chemical, physical, and mechanical attributes of the substrate and adhesive determines the ability to form a joint and the resistance of the joint to failure.39 Achievement of such interfacial molecular contact is a necessary first step in the formation of strong and stable adhesive joints. Inherent in the formation of an optimal adhesive bond is the ability of the adhesive to wet and spread on the adherends being joined. Good wetting usually occurs with solids that demonstrate high surface energy. Adhesives should exhibit low viscosities to form low contact angles, thus increasing their wetting capabilities.40
Once wetting is achieved, intrinsic adhesive forces are generated across the interface through mechanisms of mechanical interlocking, adsorption, diffusion, or any one of their combinations. Mechanical interlocking occurs when adhesive flows into pores in the adherend surface or around projections on the surface. In adsorption, adhesive molecules adsorb onto a solid surface and bonds to it. This process may involve the chemical bonding between the resin (adhesive) and the inorganic or organic elements (adherend) of the tooth structure. Diffusion involves a mechanical or chemical bonding between polymer molecules (resin) and a precipitation of substances on the tooth surface. Most often, more than one of these mechanisms play a role in achieving the desired level of adhesion for various types of adhesive and adherend.39
The bonded restorative complex includes the outer layers of the substrate, the adhesive layer, and the restorative material. The integrity of the adhesive bonded interface is subject to failure arising from defects along the interface that can result in adhesive joint failure from debonding from crack formation and propagation. These defects come from trapped air voids, voids formed from solvent evaporation, areas of poor wetting, bubbles within the adhesive, curing shrinkage pores, areas of interfacial contamination, and excess moisture contamination.39 Agitating the adhesive during placement and etching may reduce pore formation. A restorative material properly joined to the tooth substrate is able to provide an improved marginal seal40 while reducing marginal contraction gaps, microleakage, marginal staining, and caries; restoration retention from a durable interfacial adhesion between tooth and biomaterial40; and a reduction of stress at the tooth-restorative interface.41 Biomechanically, this reinforces tooth structure, and biologically, it preserves tissues, seals dentin tubules, and provides long-term functional success.41-44
A durable interfacial adhesion between the tooth and biomaterial requires a clean surface of the substrate, a low contact angle that allows the adhesive to spread over the entire surface of the substrate, and an optimal internal adaptation of the biomaterial to the substrate. Good wetting allows an intimate approximation of the material with the substrate without the entrapment of air pockets, which can result in adhesive failure. In addition, the interface should have a sufficient physical, chemical, and mechanical strength to resist stress from polymerization or occlusal forces and a sufficient degree of cure of the adhesive.38,39 Furthermore, the clinician should have an understanding of the morphologic, histologic and physiologic characteristics of the substrate (enamel and dentin) and the strategies for their biomodification to achieve optimal adhesion.
Biomodification and Adhesion
The biomodification of enamel and dentin by buffered acids facilitates interdiffusion of resins into the dental tissue substrates and has been the standard clinical procedure in adhesive dentistry since the 1960s.18 Acids can remove the smear layer either completely or partially. This raises the surface energy of the tissue, reduces the mineral content of the substrates, and creates microretentive areas that subsequently can be infiltrated by primers and bonding resins.
The mechanism of adhesion is similar for enamel and dentin—a micromechanical entanglement of monomers into the enamel microporosities or collagen interfibrillar spaces created by acid dissolution of mineralized tissues.45,46 When evaluating restorative success, the marginal integrity achieved by this procedure becomes a priority since an intact restorative–tooth interface is essential to the exclusion of bacteria and the interfacial hydrodynamic equilibrium of the dentino-pulpal complex (Figure 8 through Figure 11).
Acid etching of enamel has become a standard procedure for resin-bonded interfaces because of its demonstrated ability to obtain a durable, effective micromechanical bond of 20 MPa or more between resin and tooth enamel.47 The acid-etch technique has provided an ideal surface for bonding to enamel by using 30% to 40% phosphoric acid.48
For successful bonding to dentin, one of two different adhesive protocols may be used. The total-etch (etch-and-rinse) protocol requires the application of acids that decalcify the surface layer of dentin. The acid removes the smear layer and opens the dentinal tubules, increases dentinal permeability, and decalcifies the intertubular and peritubular dentin. The removal of the mineralized tissues (ie, hydroxyapatite crystals) leaves a network of collagenous fibrils exposed, which overlay the deeper, decalcified dentin.49,50
The self-etching primer protocol concurrently reprecipitates the smear layer while infiltrating the decalcified dentin by an acidic monomer. This technique permits the simultaneous infiltration of the collagen fibers and decalcification of the inorganic component to the same depth in dentin, thus minimizing the risk of incomplete penetration of adhesive monomers into the demineralized dentin. Additionally, this infiltration prevents the collapse of the collagen fibers that can occur after conditioning and drying with the total-etch technique. The resin may slightly infiltrate the smear layer and the dentin and copolymerize.51
In comparison to total-etch adhesives, the self-etch adhesives do not allow a discrepancy between the depth of demineralization and depth of resin infiltration because both processes occur simultaneously.45 Therefore, the potential for postoperative sensitivity may be less with self-etch systems because the smear plugs are not removed before the application of the adhesives. However, one clinical study reported that there was no difference between total-etch and self-etch adhesives.52
Selective etching is another adhesive protocol that uses acid etching of enamel and self-etching of the dentin. This technique incorporates the benefits of the total- and self-etching protocols. The selective demineralization of the dentin reduces the potential for sensitivity while providing superior and more predictable bond strengths to dentin. The selective demineralization of the enamel improves bond strength by creating a more pronounced and retentive etching pattern in enamel, while promoting higher marginal adaptation and improving bond durability.
All of these adhesive protocols permit the formation of a resin-reinforced zone, (ie, the resin-infiltrated layer or hybrid layer) that is the primary bonding mechanism of many current adhesive systems.49,50 This hybridization of the exposed dentin with an adhesive system is considered by some to be the most effective way of protecting this pulp-dentin interface, and bonding the resin composite to the tooth structure provides resistance to microleakage and retention of the restoration. Since the adhesive layer may absorb polymerization shrinkage stress of the resin composite hybridization allows internal adaptation for stress relief at the restorative interface between resin composite and the dentin, while eliminating sensitivity.53,54 This process results in improved marginal and interfacial adaptation with reduced gap formation.
Stresses at the Restorative–Tooth Interface
The integrity of the bond and marginal adaptation to the tooth structure are critical for clinical success in composite restorations.55 The restorative–tooth interface is constantly subjected to stress and strain imposed by polymerization shrinkage forces, thermal changes, and functional occlusal loads. These stresses may be the mechanism for the clinical challenges with adhesive restorations in clinical dentistry.56 These challenges include inadequate marginal adaptation, microleakage, marginal breakdown and secondary caries, fractures, staining, postoperative sensitivity, and potential pulpal irritation.26,56-58
Polymerization Shrinkage vs Adhesion
Before a restoration is subjected to functional loads and thermal strains, there is an initial interfacial stress developed during polymerization of the restorative materials and adhesion to tooth structure.59 In a restorative technique using resin composites, the polymerization reaction of the resin matrix phase could compromise dimensional stability.56 Therefore, a comprehensive understanding of the complex interplay between polymerization shrinkage and adhesion is necessary. The conversion of the monomer molecules into a polymer network is accompanied with a closer packing of the molecules, leading to bulk contraction.60 Alternatively, when a curing material is bonded on all sides to rigid structures, bulk contraction occurs and shrinkage must be compensated for by flow of the composite resin. If flow does not occur (related to modulus development of the composite, filler load, curing light power, etc), then increased stress, tooth flexure, or gap formation can occur at the composite–adhesive–tooth interface.56
Polymerization shrinkage or curing contraction is the volumetric shrinkage of the resin composite during polymerization.32 The cross-linking of resin monomers into polymers is responsible for an unconstrained volumetric shrinkage of 2% to 5 %.61 During the polymerization reaction, the visco-elastic behavior of the composite changes from viscous to viscous-elastic to elastic. Although stress development is nonexistent in the viscous stage, in the visco-elastic stage stresses can partly be relieved by flow and elastic strain.62
The moment at which the material can no longer provide viscous flow to keep up with the curing contraction is referred to as the gel point.56 When the composite material develops an elastic modulus, a volumetric polymerization contraction results in shrinkage stresses. The shrinkage stresses are transferred to the surrounding tooth structure, because the volumetric changes are restricted.62 The uncompensated forces may exceed the bond strength of the tooth-restoration interface, resulting in a gap formation from a loss of adhesion.63 Factors that influence polymerization shrinkage include: type of resin,32 filler content of the composite,32,64,65 elastic modulus of the material,65 curing characteristics,66 water sorption,67-69 cavity configuration,26 and the intensity of the light used to polymerize the composite (Figure 12).62,70,71 By understanding this complex mechanism between polymerization shrinkage and adhesion, the clinician can select restorative methods and materials for the management of stress at the restoration–tooth interface for each individual clinical situation.
Restorative Methods for Management of Stress
A final component of the adhesive design concept is technique. The disparate adoption of the latest materials and technologies may not lead to clinical success as much as the technique.18 Kopperud and colleagues suggest that a clinicians’ skill in performing adhesive protocol is important to increasing the longevity of composite restorations.72 To overcome the clinical challenges in direct posterior composite restorations, several clinical restorative methods for the management of stress at the restoration–tooth interface should be considered when selecting restorative materials that are subject to shrinkage stress. These undesirable effects can be managed and minimized with the following methods: utilizing low-shrinkage composite resins,73 the use of low-modulus liners,74,75 the use of an intermediate layer of glass ionomer,76-78 selective bonding in appropriate cavity configurations,79,80 use of lower modulus resin composites, controlling the curing-light intensity,75,81,82 use of condensation and polymerization tips,83,84 reducing the volume of composite restorative material with the use of inlay techniques,18,33 and layering of small increments of resin composites (Figure 13 and Figure 14).85-90
Placement Procedures and Techniques
A fundamental requirement for successful bonding of directly placed adhesive restorations requires isolation of the tooth. The best means of moisture control is the dental dam. Numerous studies report microleakage, reduced adhesion, and bond strength reduction from contamination of enamel with saliva, moisture, and moisture contamination from blood and crevicular fluid.91
Incremental layering also improves the operator’s control of resin placement, marginal adaptation, polymerization of the restorative material, and bond formation (Figure 15 through Figure 19). Additionally, stratification provides control of overhangs in the lateral margins prior to curing, reduces the effects of polymerization shrinkage, allows the orientation of the curing light beam according to the position of each composite layer, and allows the placement of optimal anatomical contours of the restoration (Figure 20 through Figure 22).92,93 It is important to have the flat plane of the curing light tip diameter parallel to the plane of the material, causing shadowless exposures.
Conclusion
Securing and maintaining a relatively stress-free restorative–tooth interface contributes greatly to restorative success. The adhesive design concept describes this rationale for the preparation and placement of adhesive restorations. This concept explains that the preparation design can be, and is, influenced by the selection of the restorative biomaterial.94 It also provides insight into the interplay between adhesion and polymerization shrinkage within these adhesive materials and how they can be influenced by placement techniques and adhesive protocols. Thus, proper selection and use of biomaterials with thorough and accurate adhesive protocols and precise placement techniques can directly influence the longevity of these restorations. As the industry continues to develop improved methods and materials, the clinician should consider using the aforementioned adhesive design concept while exploring new products and techniques.
References
1. Rubinstein S, Nidetz AJ. Posterior direct resin-bonded restorations: still an esthetic alternative. J Esthet Dent. 1995;7(4):167-173.
2. Jackson RD, Morgan M. The new posterior resins and a simplified placement technique. J Am Dent Assoc. 2000;131(3):375-383.
3. Bichacho N. Direct composite resin restorations of the anterior single tooth: clinical implications and practical applications. Compend Contin Educ Dent. 1996;17(8):796-802.
4. Dietschi D, Scampa U, Campanile G, Holz J. Marginal adaptation and seal of direct and indirect Class II composite resin restorations: an invitro evaluation. Quintessence Int. 1995;26(2):127-138.
5. Full CA, Hollander WR. The composite resin restoration: a literature review. Part I. Proper cavity preparation and placement techniques. ASDC J Dent Child. 1993;60(1):48-51.
6. Dietschi D, De Siebenthal G, Neveu-Rosenstand L, Holz J. Influence of the restorative technique and new adhesives on the dentin marginal seal and adaptation of resin composite Class II restorations: an in vitro evaluation. Quintessence Int. 1995;26(10):717-727.
7. Eames WB, Strain JD, Weitman RT, Williams AK. Clinical comparison of composite, amalgam, and silicate restorations. J Am Dent Assoc. 1974;89(5):1111-1117.
8. Mazik CA. Simplified occlusal anatomy for posterior composites. J Esthet Dent. 1992;4(1):8-10.
9. Feinman RA. The plunging ball technique: Class II direct composite resins. Pract Periodontics Aesthet Dent. 1992;4(5):43-48.
10. Lacy AM. A critical look at posterior composite restorations. J Am Dent Assoc. 1987;114(3):357-362.
11. Lyons K. Alternatives to amalgam. N Z Dent J. 1997;93(412):47-50.
12. Dickerson WG. A functional and aesthetic direct resin technique. Pract Periodontics Aesthet Dent. 1991;3(7):43-47.
13. Leinfelder KF. A conservative approach to placing posterior composite resin restorations. J Am Dent Assoc. 1996;127(6):743-748.
14. Bichacho N. The centripetal build-up for composite resin posterior restorations. Pract Periodontics Aesthet Dent. 1994;6(3):17-23; quiz 24.
15. Bayne SC, Heymann HO, Sturdevant JR, Wilder AD, Sluder TB. Contributing co-variables in clinical trials. Am J Dent. 1991;4(5):247-250.
16. Terry DA, Leinfelder KF, James A. A nonmechanical etiology: The adhesive design concept. Pract Proced Aesthet Dent. 2006;18(6):385-391.
17. Leinfelder KF. Using composite resin as a posterior restorative material. J Am Dent Assoc. 1991;122(4):65-70.
18. Terry DA, Geller W. Esthetic & Restorative Dentistry: Material Selection & Technique. 2nd ed. Chicago, IL: Quintessence Publishing; 2013.
19. Liebenberg WH. Assuring restorative integrity in extensive posterior resin composite restorations: pushing the envelope. Quintessence Int. 2000;31(3):153-164.
20. Taylor DF, Bayne SC, Sturdevant JR, Wilder AD. Restoration width and complexity effects on posterior composite wear. J Dent Res. 1989;68 (abstract, special issue A):186.
21. Leinfelder KF. Composite resins in posterior teeth. Dent Clin North Am. 1981;25(2):357-364.
22. Ferrari M, Kugel G. Handling characteristics of resin composites in posterior teeth. Compend Contin Educ Dent. 1998;19(9):879-882, 884, 886 passim; quiz 894.
23.Dietschi D, Spreafico R. Adhesive Metal-Free Restorations: Current Concepts for the Esthetic Treatment of Posterior Teeth. Berlin, Germany: Quintessence; 1999.
24. Sabiston CB Jr. Etiology of cracked teeth: a review and proposal. Iowa Dent J. 1994;80(4):13-14.
25. Roulet JF. Degradation of Dental Polymers. 1st ed. Basel, Switzerland: S. Karger AG; 1987.
26. Feilzer AJ, De Gee AJ, Davidson CL. Setting stress in composite resin in relation to configuration of the restoration. J Dent Res. 1987;66(11):1636-1639.
27. Davidson CL, de Gee AJ. Relaxation of polymerization contraction stresses by flow in dental composites. J Dent Res. 1984;63(2):146-148.
28. Terry DA, Leinfelder KF. Managing stress with composite resin, Part 1: The restorative-tooth interface. Dent Today. 2006;25(12):98, 100-4; quiz 104.
29. Hood JA. Biomechanics of the intact, prepared and restored tooth: some clinical implications. Int Dent J. 1991;41(1):25-32.
30. Lutz FU, Krejci I, Oddera M. Advanced adhesive restorations: the post-amalgam age. Pract Periodontics Aesthet Dent. 1996;8(4):385-94; quiz 398.
31. Lutz F. State of the art of tooth-colored restoratives. Oper Dent. 1996;21(6):237-248.
32. Quellet D. Considerations and techniques for multiple bulk-fill direct posterior composites. Compend Contin Educ Dent. 1995;16(12):1212, 1214-1216, passim; quiz 1226.
33.Terry DA. Natural Esthetics with Composite Resin. 1st ed. Mahwah, NJ: Montage Media Corporation; 2004.
34. Wilson NH, Dunne SM, Gainsford ID. Current materials and techniques for direct restorations in posterior teeth. Part 2: Resin composite systems. Int Dent J. 1997;47(4):185-193.
35. Sturdevant CM, Roberson TM, Heymann HO, Sturdevant JR. The Art and Science of Operative Dentistry. 3rd ed. St Louis, Missouri: Mosby-Year Book; 1995.
36. Isenberg BP, Leinfelder KF. Efficacy of beveling posterior composite resin preparations. J Esthet Dent. 1990;2(3):70-73.
37. Small BW. Direct posterior composite restorations--state of the art 1998. Gen Dent. 1998;46(1):26-32.
38. Anusavice KJ. Structure of matter and principles of adhesion. 10th ed. In: Phillips’ Science of Dental Materials. Philadelphia, PA: W.B. Saunders Company; 1996.
39. Powers JM, Sakaguchi RL, eds. Craig’s Restorative Dental Materials. 12th ed. St. Louis, Missouri: Mosby Elsevier; 2006.
40. Armstrong SR, Boyer DB, Keller JC. Microtensile bond strength testing and failure analysis of two dentin adhesives. Dent Mater. 1998;14(1):44-50.
41. Goracci G, Mori G. Esthetic and functional reproduction of occlusal morphology with composite resins. Compend Contin Educ Dent. 1999;20(7):643-648; quiz 650.
42. Santos J, Bianchi J. Restoration of severely damaged teeth with resin bonding systems: case reports. Quintessence Int. 1991;22(8):611-615.
43. Van Meerbeek B, Vanherle G, Lambrechts P, Braem M. Dentin- and enamel-bonding agents. Curr Opin Dent. 1992;2:117-127.
44. Eakle WS. Fracture resistance of teeth restored with class II bonded composite resin. J Dent Res. 1986;65(2):149-153.
45. Perdigão J, Geraldeli S. Bonding characteristics of self-etching adhesives to intact versus prepared enamel. J Esthet Restor Dent. 2003;15(1):32-41; discussion 42.
46. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265-273.
47. Phillips RW, Avery DR, Mehra R, Swartz ML, McCune RJ. Observations on a composite resin for Class II restorations: three-year report. J Prosthet Dent. 1973;30(6):891-897.
48. Perdigão J, Frankenberger R, Rosa BT, Breschi L. New trends in dentin/enamel adhesion. Am J Dent. 2000;13(Spec No):25D-30D.
49. Swift EJ Jr, Perdigão J, Heymann HO. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int. 1995;26(2):95-110.
50.Van Meerbeek B, Inokoshi S, Braem M, Lambrechts P, Vanherle G. Morphological aspects of the resin-dentin interdiffusion zone with different dentin adhesive systems. J Dent Res. 1992;71(8):1530-1540.
51. Eick JD, Robinson SJ, Chappell RP, et al. The dentinal surface: Its influence on dentinal adhesion. Part III. Quintessence Int. 1993;24(8):571-582.
52. Perdigão J, Geraldeli S, Hodges JS. Total-etch versus self-etch adhesive: effect on postoperative sensitivity. J Am Dent Assoc. 2003;134(12):1621-1629.
53. Van Meerbeek B, Perdigão J, Lambrechts P, Vanherle G. The clinical performance of adhesives. J Dent. 1998;26(1):1-20.
54. Lindberg A, van Dijken JW, Hörstedt P. Interfacial adaptation of a Class II polyacid-modified resin composite/resin composite laminate restoration in vivo. Acta Odontol Scand. 2000;58(2):77-84.
55. Bouschlicher MR, Cobb DS, Boyer DB. Radiopacity of compomers, flowable and conventional resin composites for posterior restorations. Oper Dent. 1999;24(1):20-25.
56. Davidson CL, Feilzer AJ. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J Dent. 1997;25(6):435-440.
57. Kemp-Scholte CM, Davidson CL. Marginal sealing of curing contraction gaps in Class V composite resin restorations. J Dent Res. 1988;67(5):841-845.
58. Bausch JR, de Lange K, Davidson CL, Peters A, de Gee AJ. Clinical significance of polymerization shrinkage of composite resins. J Prosthet Dent. 1982;48(1):59-67.
59. Labella R, Lambrechts P, Van Meerbeek B, Vanherle G. Polymerization shrinkage and elasticity of flowable composites and filled adhesives. Dent Mater. 1999;15(2):128-137.
60. Loshaek S, Fox TG. Cross-linked polymers. I . Factors influencing the efficiency of cross-linking in copolymers of methyl methacrylates and glycol methacrylates. J Amer Chem Soc. 1953;75:3544-3550.
61. Ferracane JL. Using posterior composites appropriately. J Am Dent Assoc. 1992;123(7):53-58.
62. Feilzer AJ, Dooren LH, de Gee AJ, Davidson CL. Influence of light intensity on polymerization shrinkage and integrity of restoration-cavity interface. Euro J Oral Sci. 1995;103(5):322-326.
63. Davidson CL, de Gee AJ, Feilzer A. The competition between the composite-dentin bond strength and the polymerization contraction stress. J Dent Res. 1984;63(12):1396-1399.
64. Munksgaard EC, Hansen EK, Kato H. Wall-to-wall polymerization contraction of composite resins versus filler content. Scand J Dent Res. 1987;95(6):526-531.
65. Iga M, Takeshige F, Ui T, et al. The relationship between polymerization shrinkage measured by a modified dilatometer and the inorganic filler content in light-cured composites. Dent Mater. 1991;10:38-45.
66. Krejci I, Lutz F. Marginal adaptation of Class V restorations using different restorative techniques. J Dent. 1991;19(1):24-32.
67. Alster D, Feilzer AJ, de Gee AJ, et al. The dependence of shrinkage stress reduction on porosity concentration in thin resin layers. J Dent Res. 1992;71(9):1619-1622.
68. Fan PL, Edahl A, Leung RL, Stanford JW. Alternative interpretations of water sorption values of composite resins. J Dent Res. 1985;64(1):78-80.
69. Soltesz U, Bath P, Klaiber B. Dimensional behavior of dental composites due to polymerization shrinkage and water sorption. In: Christel P, Meunier A, Lee AJC, eds. Biological and Biomechanical Performance of Biomaterials. Amsterdam: Elsevier;1986:123-128.
70. Unterbrink GL, Muessner R. Influence of light intensity on two restorative systems. J Dent. 1995;23(3):183-189.
71. Uno S, Asmussen E. Marginal adaptation of a restorative resin polymerized at reduced rate. Scand J Dent Res. 1991;99(5):440-444.
72. Kopperud SE, Tveit AB, Gaarden T, et al. Longevity of posterior dental restorations and reasons for failure. Eur J Oral Sci. 2012;120(6):539-548.
73. Asmussen E. Composite restorative resins. Composition versus wall-to-wall polymerization contraction. Acta Odontol Scand. 1975;33;337-343.
74. Braga RR, Ferracane JL. Alternatives in polymerization contraction stress management. Crit Rev Oral Biol Med. 2004;15(3):176-184.
75. Cho NY, Ferracane JL, Lee IB. Acoustic emission analysis of tooth-composite interfacial debonding. J Dent Res. 2013;92(1):76-81.
76. Olliveira LC, Duarte S Jr., Araujo CA, Abrahão A. Effect of low-elastic modulus liner and base as stress-absorbing layer in composite resin restorations. Dent Mater. 2010;26(3):159-169.
77. Braga RR, Hilton TJ, Ferracane JL. Contraction stress of flowable composite materials and their efficacy as stress-relieving layers. J Am Dent Assoc. 2003;134(6):721-728.
78. Güray Efes B, Yaman BC, Gümüstas B, Tiryaki M. The effects of glass ionomer and flowable composite liners on the fracture resistance of open-sandwich class II restorations. Dent Mater J. 2013;32(6):877-882.
79. Lutz F, Krejci I, Barbakow F. Quality and durability of marginal adaptation in bonded composite restorations. Dent Mater. 1991;7(2):107-113.
80. Bertolotti R. Posterior composite technique utilizing directed polymerization shrinkage and a novel matrix. Prac Periodont Aesthet Dent. 1991;3(4):53-58.
81. Obici AC, Sinhoreti MA, De Goes MF, et al. Effect of the photo-activation method polymerization shrinkage of restorative composites. Oper Dent. 2002;27(2):192-198.
82. Kays BT, Sneed WD, Nuckles DB. Microhardness of class II composite resin restorations with different matrices and light positions. J Prosthet Dent. 1991;65(4):487-490.
83. Dietschi D, Magne P, Holz J. Recent trends in esthetic restorations for posterior teeth. Quint Int. 1994;25(10):659-677.
84. Jorgensen K, Hisamitsu H. Class 2 composite restorations: Prevention in vitro of contraction gaps. J Dent Res. 1984;63(2):141-145.
85. Park J, Chang J, Ferracane JL, Lee IB. How should composite be layered to reduce shrinkage stress: incremental or bulk filling? Dent Mater. 2008;24(11):1501-1505.
86. Usha HL, Kumari A, Mehta D, et al. Comparing microleakage and layering methods of silorane-based resin composite in class V cavities using confocal microscopy: An in vitro study. J Conserv Dent. 2011;14(2):164-168.
87. Kwon Y, Ferracane JL, Lee IB. Effect of layering methods, composite type, and flowable liner on the polymerization shrinkage stress of light cured composites. Dent Mater. 2012;28(7):801-809.
88. Crim GA. Microleakage of three resin placement techniques. Am J Dent. 1991;4(2):69-72.
89. Opdam NJ, Feilzer AJ, Roeters JJ, Smale I. Class I occlusal composite resin restorations: in vivo post-operative sensitivity, wall adaptation, and microleakage. Am J Dent. 1998;11(5):229-234.
90. Vandewalle KS, Ferracane JL, Hilton TJ, et al. Effect of energy density on properties and marginal integrity of posterior resin composite restorations. Dent Mater. 2004;20:96-106.
91. Barghi N, Knight GT, Berry TG. Comparing two methods of moisture control in bonding to enamel: a clinical study. Oper Dent. 1991;16(4):130-135.
92. Kovarik RE, Ergle JW. Fracture toughness of posterior composite resin fabricated by incremental layering. J Prosthet Dent. 1993;69(6):557-556.
93. Versluis A, Douglas WH, Cross M, Sakaguchi RL. Does an incremental filling technique reduce polymerization shrinkage stresses? J Dent Res. 1996;75(3):871-878.
94. Terry DA, Geller W. Selection defines design. J Esthet Restor Dent. 2004;16(4):213-225.
About the Authors
Douglas A. Terry, DDS
Adjunct Professor, Dept. of Restorative Sciences University of Alabama at Birmingham
Professor Emeritus, Dept. of Conservative Dentistry and Endodontics
V.S. Dental College & Hospital, Rajiv Gandhi University of Health Sciences
Bangalore, India
Private Practice
Houston, Texas
John M. Powers, PhD
Clinical Professor of Oral Biomaterials
The University of Texas School of Dentistry
Houston, Texas