You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Bruxism is defined as a diurnal and nocturnal parafunctional activity that includes clenching, bracing, gnashing, and grinding of teeth.1 The damage from bruxism is a reality in the everyday practice of dentistry and yet there is a great deal of confusion and controversy. Dental professionals do not even agree on the relative number of people who brux. The estimates range from 5% to 95% of the population.2-4 Many dentists focus on the patients who present with wear to determine rates of bruxism. Tooth wear, however, is a poor indicator of bruxism since attrition may play only a small role in tooth destruction and may not be indicative of an ongoing problem.5 The smaller estimates limit the patients to sleep bruxers. Sleep bruxism (SB) is the grinding or clenching of the teeth during sleep, usually associated with sleep arousals.6 To date, the pathophysiology for SB has not been definitively determined. Research points to neurochemistry, autonomic system, and sleep arousals as possible triggers.7,8 This article will restrict its focus to a unique subset of the bruxing population: the sleep bruxer. This group is extremely destructive to their teeth and systemic health. Additionally, this article will discuss the significance of the bruxism triad: sleep bruxism, sleep disturbance, and sleep-related gastroesophageal reflux.
Tooth Wear
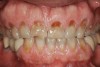
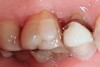
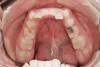
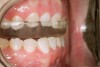
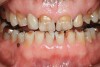
Tooth wear is described in the literature as the loss of the constitution of the tooth and has been classified as being caused by attrition, abrasion, erosion, or a combination of these factors.9 As it relates to SB, tooth wear is reported to additionally cause tooth mobility, temperature hypersensitivity, and tooth fracture.10 While sleep bruxers and non-bruxers displayed significantly different amounts of wear over time, the contribution of tooth-on-tooth attrition to this wear is still controversial.11 It has been postulated that much of the wear could be erosion rather than attrition. Interestingly, those two factors are interwoven in the bruxism triad patient, magnifying the wear in this patient population (Figure 1).
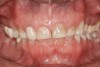
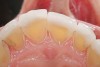
The study of tooth wear is appropriately described as tribology: the science of interacting surfaces in relative motion and associated issues of lubrication, friction, and wear. Teeth sliding over each other are affected by a complex tribological interaction. Friction is encountered whenever there is relative motion between contacting surfaces, and it always opposes the motion. As no surface is perfectly smooth, when the teeth make contact, even under light load, it may produce the loss of tooth structure. When non-roughened surfaces contact, their coefficient of friction decreases dramatically if a lubricant is introduced. Tribology would suggest that a decrease in oral lubrication coupled with tooth-on-tooth contact would introduce friction and, thus, wear.12 In addition, elements that increase the surface roughness (eg, erosion) would unavoidably increase wear (Figure 2).
Intraoral lubrication is provided primarily by saliva. It also lubricates and buffers the esophagus and decreases the risk of aspiration.12 Salivary flow follows a daily circadian variation and is significantly lower at night. During sleep, deglutination is episodic, with long periods without swallowing. Daytime swallowing averages 25 to 60 times per hour and only two to nine times at night.13 Salivary flow and buffering capacity vary between individuals and may be insufficient to prevent frictional tooth damage.14 Swallowing is almost exclusively associated with microarousals (MA) during light sleep.13 These MAs play an important role in sleep bruxism.
Reduced lubrication, erosive friction, and contact time play significant roles in tribologic wear of teeth in sleep bruxers. Bruxing force is not as important as previously thought. For years the profession has accepted that SB patients can exert up to six times as much force on their teeth at night than normal subjects.15 It has provided dentists a simple explanation to our patients as to why their teeth wear and restorations break. Interestingly, the facts simply do not support that conclusion. Gibbs et al wrote that the bite strength in some bruxer-clenchers can be as much as six times that of the non-bruxer.15 The study evaluated daytime bite strength, not nocturnal bruxing force. It also did not provide groups for bruxing subjects and a non-bruxing control. In fact, the conclusion of the study should have read that only one patient in the study during the day could produce six times greater force on biting than the average dentate patient. Studies monitoring muscle activity during sleep have confirmed that the elevator muscles are rarely, if ever, contracted to their maximum. In fact, when the EMG levels are evaluated during nocturnal bruxing activity, the muscle response is only half of the maximum voluntary contraction.16,17 Only 66% of the bruxism episodes are at a force level equal to or greater than the force generated during chewing. While it is true that patients with greater jaw muscle size may generate more total force with the same EMG activity, the amount of force is well within daily norms and definitely not six times greater.
Sleep Stages and Arousal Response
Good-quality sleep is important for physical recovery, biochemical refreshment, memory consolidation, and emotional regulation.18 A typical sleep cycle is 90 to 110 minutes of sleep with three to five cycles per night. Sleep is composed of two distinct states: non-REM (quiet sleep) and REM (active sleep). There are four stages of non-REM sleep. Stages 1-2 are light sleep and Stages 3-4 are deep sleep. In the first third of total sleep, non-REM Stage 3-4 is the longest stage and decreases or disappears in the last third. REM sleep increases in the last third. While dreams may occur in any stage of sleep, they are more common and more vivid in REM sleep.20
A micro-arousal (MA) is a shift in sleep occurring during deeper sleep. These 3- to 10-second rises in EEG activity create an increase in heart rate and muscle tone. MAs occur 8 to 15 times in an hour. Bruxism is an oromotor manifestation secondary to MA. Eighty-six percent of bruxism occurs in non-REM Stage 2 sleep and MA.19 A smaller percentage of bruxism events occur in REM sleep.20 More destructive bruxers, however, have a greater number of episodes and time of bruxism in REM sleep than controls.21
Sleep Disturbances
Respiratory disturbances during sleep fall into three categories: snoring, upper airway resistance syndrome (UARS), and sleep apnea-hypopnea syndrome. Snoring is defined as sounds produced in the upper airway from soft tissue vibration induced by air turbulence. It is reported in 40% of men and 24% of women. The incidence is 10% in children.22 Snoring is a risk factor for obstructive sleep apnea (OSA). A medical consultation is appropriate before making an oral appliance for snoring and is mandatory when snoring is accompanied by daytime sleepiness, insomnia, sleep disruption, or hypertension.
UARS is an increased inspiratory effort creating increased MAs and narrowing of the pharynx without oxygen desaturation below 4%.23 It is characterized by repetitive partial collapse of the upper airway during sleep. UARS have been linked with increased bruxism, headaches, and TMD, and are thought to be an intermediate form of disorder between snoring and OSA.22
Apnea is the repetitive absence of ventilation with cessation of breathing for 10 seconds and oxygen desaturation exceeding 4%. Sleep apnea may be obstructive sleep apnea (most common) resulting from a blockage of the upper airway or central sleep apnea demonstrated by no chest movements resulting from a lack of neural drive.18 Patients may have both types simultaneously. Hypopnea is a decrease in airflow of more than 50% or a decrease of airflow by 30% with an oxygen desaturation of more than 3%. The level of OSA is related to the number of apnea-hypopnea events per hour of sleep. The apnea-hypopnea index (AHI) considers persons with 5 to 15 events per hour of sleep as mild, 15 to 30 as moderate, and > 30 as severe. The severity of sleep apnea is judged by a composite of the symptoms including AHI, daytime sleepiness, and % desaturation. Risk factors for OSA include being male, overweight, over 40, large neck size, large tonsils, and family history (Figure 3).24 It is estimated that 1 in 5 adults has at least mild obstructive sleep apnea (OSA) and 1 in 15 has at least moderate.25 Unlike the bruxism prevalence, OSA increases with age and can affect 70% of men and 56% of women over the age of 65, a three-fold increase from middle age.26 While beyond the scope of this article, OSA is a risk factor for hypertension, cardiovascular morbidity, and daytime sleepiness to name just a few.25
Sleep Bruxism
A sleep bruxer is different than a patient who occasionally bruxes during sleep. By definition, a sleep bruxer must have > 4 episodes of bruxing per hour of sleep, > 25 bruxing bursts per hour, and at least one episode per night must make noise.27 SB is higher in children and decreases with age. SB occurs in up to 30% of children from 5 to 6 years old, 13% in respondents 18 to 29 years of age, and decreasing to 3% in patients over 60 years old.28 Unlike stress-triggered bruxing subjects, sleep bruxing episodes are unrelated to experienced and anticipated stress.29 In addition, SB has little variability in the bruxing episodes and bursts per hour of sleep over months and years.30 In moderate to severe SB, grinding was present every night.31
Sleep bruxism episodes are related to disturbances in sleep. Kato and colleagues induced MAs during the sleep of sleep bruxers and controls.32 Tooth grinding followed the experimentally induced MA in more than 71% of the trials. Interestingly, this reaction was only produced in sleep bruxism patients and never in controls, indicating a heightened responsiveness to sleep arousals. Therefore, anything that induces a greater number of MAs has the ability to increase the amount of tooth grinding in these subjects.
Airway
Researchers observe that the frequency of sleep apnea increased as the frequency of bruxism increased. Given the link between MA and SB, it may be more correctly stated that the frequency of SB increases with an increase in sleep apnea-induced arousals.22,33 At least one third of patients with bruxism in the general population may also have sleep-disordered breathing conditions such as sleep apnea, periodic leg movement during sleep, and headache.22,34 The rate of OSA may be as high as 30% in a TMD population.18 In addition to OSA, close to 50% of UARS patients complain of bruxism.22 Prospective evaluations and case studies of SB and apnea indicate that bruxism events may be directly correlated to apnea episodes.35 While a causal relationship cannot be made, obstructive sleep apnea (OSA) syndrome has been called the highest risk factor for tooth grinding during sleep.36
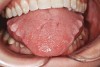
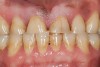
Bruxism and airway appear to be related to the patient’s attempt to develop a patent airway during a desaturation episode. The majority of SB episodes occurs in a supine position and may be associated with either a reduction in the airway passage or increase in its resistance. During resumption of ventilation following apnea, a co-activation of both jaw-opening and jaw-closing muscles produce dilation of the upper airway. This permits a rise in inspiratory flow and reduces upper airway resistance.37 It has been reported that 99% of all rhythmic masticatory muscle activities were associated with a change in the respiratory amplitude and frequency.19 Changes in lateral tongue contours, long associated with nocturnal bruxers, can now be explained. The patient attempts to provide a patent airway by activating the tongue muscles and forcing the tongue off the airway and against the teeth (Figure 4).
Bruxism is greatest in 5- to 6-year-olds and slowly declines with age. Adenoid tonsil hypertrophy in the 5- to 6-year-old patient may account for the airway obstruction and, thus, a greater incidence of bruxism.28 As the airway improves with age, the bruxism decreases in the general population but in the triad group it continues. If bruxism is a reflexive mechanism to improve or protect an airway, then greater bruxism could lower the apnea. Sjöholm concluded that there is limited correlation between bruxing and apnea because mild apnea patients had more bruxing events than moderate apnea patients.38 However, if bruxism is a protective reflex, clinicians might be able to predict the possibility in a young patient population that bruxism would be linked to less severe apnea. Aggressive bruxism in 20- to 40-year-old subjects may simply be an attempt to open their airway. As the sleep bruxer ages, their neurochemical ability to brux decreases, or it cannot overcome the additional airway obstructions due to a loss of muscle tone, weight gain, etc.
GERD
Gastroesophageal reflux disorder (GERD) is a medical condition where the stomach contents leak to the esophagus. It affects approximately 40% of Americans.39 GERD is commonly associated with heartburn or indigestion, although > 50% of people complaining of frequently clearing their throat, hoarseness, or trouble swallowing were found to have silent GERD. Poor quality of sleep may be the sole presentation of silent GERD in asymptomatic subjects.40 Medical consultation is recommended. Differential diagnosis would include peptic ulcer, angina, and Barrett esophagus (possible precursor to adenocarcinoma).41
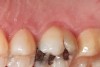
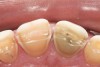
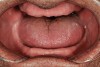
Some of the acid content of the stomach may reach the oral cavity. This is an extremely destructive acid with a pH of 1 to 2. In comparison, dietary acids are greater than pH 3. The most common site for damage is the palatal surface of the maxillary molars (Figure 5 and Figure 6). Reflux symptoms present mostly in a supine position. The dorsum of the tongue pushes the acid to the maxillary molar palatal surface when swallowing to buffer the acid.42 While the palatal surface is the most common site of destruction, the pattern of damage will be dictated by the sleep position of the patient during the episodes. Tongue activity associated with airway patency coupled with regurgitation may also create wear on the lingual surfaces of teeth resembling bulimia but are not limited to the maxillary anteriors (Figure 7). GERD patients have a significantly higher risk of xerostomia and oral burning sensation.43 This lack of lubrication paired with acid-roughened surfaces increase the risk of frictional wear associated with sleep bruxism.
Bruxism Triad: Sleep Bruxism, Sleep Disturbance, and Sleep-Related GERD
The bruxism triad coupled with nocturnal hyposalivation or xerostomia appreciably increases the risk of frictional and erosive tooth wear.12,18 The bruxism triad is composed of arousal-induced tooth grinding, airway-associated sleep disorders, and sleep-related GERD (Figure 8 and Figure 9). While a causal relationship has not been established, significant correlation makes it important for dentists to evaluate their patient population.
Sleep bruxism is concomitant with sleep apnea. Therefore, if the level of apnea can be artificially reduced, a resultant decrease in bruxism would be anticipated. Oksenberg and Arons found that during continuous positive airway pressure (CPAP) treatment, apneas were eliminated and only a few hypopneas were seen. A complete disappearance of all bruxism events occurred.35 Mandibular advancement appliances (MAA) have reported to reduce bruxism events 50% to 83%.44 The variability appears to be related to the appliances’ ability to reduce desaturation episodes, which is more inconsistent than CPAP therapy. The MAA may also be used in SB patients without respiratory disturbances. A statistically significant reduction (39% and 47%) of SB episodes per hour was recorded with the MAA at protrusion of 25% and 75%, respectively,45 providing further evidence that improving airway patency is an important treatment strategy for bruxism even in a normal patient population.
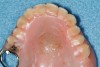
One important note is that traditional occlusal stabilizing splints may have a deleterious effect on SB-OSA patients. A prospective study of 10 mild to moderate OSA patients was conducted with multiple nights recorded with and without a stabilizing splint. Results indicated that six of the 10 subjects had a significant increase in their AHI with splint therapy.46 Further, OSA patients with complete dentures have significantly improved airways when sleeping with their dentures than without them.47 Both studies highlight that OSA patients have an improved airway when the mandible is placed and held in a protruded position. Efforts which prevent that ability may worsen the airway and increase sleep bruxism. This could account for the severity of wear found on some occlusal splints. The author has noted that patients with airway and GERD issues have notably more wear on their orthosis regardless of design (Figure 10 and 11).
GERD can be linked to the other members of the triad. Patients with GERD had higher AHI scores and shorter periods in restful Stage 2 sleep.48 Research has also demonstrated that more severe OSA was accompanied by more severe GERD.49 While the link is still controversial, one explanation is that during apnea episodes, there is an increase in negative intrathoracic pressure. This increased negative pressure could cause the gastric acids to be expelled from the stomach and into the esophagus. As the esophageal pH decreases, patients’ bruxing episodes were significantly higher. Miyawaki studied 10 SB and 10 controls presenting without GERD symptoms. Esophageal pH was monitored during an evening of sleep. Results showed that when the esophageal pH reached 4 or lower there was a simultaneous bruxism episode ending in a tonic burst representing a swallow.50 This is apparently an attempt to buffer the esophageal acid content. Interestingly, the pH 4 threshold was reached exclusively by SB patients. No control patient had an esophageal pH low enough to trigger a bruxism episode.
Proton-pump inhibitors are a group of drugs that reduce gastric-acid production. Administration of a proton-pump inhibitor to GERD-SB patients demonstrated a commensurate 40% reduction of SB episodes.50 Another family of GERD medication, the H2 blocker anti-acids, showed a reduction in MAs, respiratory disturbances, and daytime somnolence. It did not, however, reduce the AHI.51 Reducing the intrathoracic pressure with CPAP reduces GERD parameters in patients with and without OSA.51,52 Studies with MAA have not been conducted but similar results would be anticipated given their impact on the airway.
Recognizing the Triad Patient
Dentists should to be able to recognize the bruxism triad patient at all stages of life. Early diagnosis can alter poor growth, improve physical well-being, and reduce tooth wear. The following are some of the distinguishing features of each of the stages of the triad.
Childhood 3–12
Adenoid and tonsil hypertrophy produces sleep disturbances including OSA. The magnitude of hypertrophy required for obstruction is smaller in obese children compared to non-obese children.53 GERD will be found frequently in children with adenotonsillar hypertrophy and OSA and should be evaluated.54 Five-year-old children with OSA will commonly display differences in growth when compared to matched controls. This can include a mandibular posterior inclination, greater anterior face height, and retroclined incisors (Figure 12).55
Adolescent 13–19
Dentists commonly tell parents that most children “grow out of bruxism” when they reach puberty. More accurately, many children grow enough to overcome the impact of the adenoids and tonsils, or orthodontic intervention expands the palate enough to create an adequate airway. Any signs of pathologic wear due to attrition or erosion at this stage should elicit questions about sleep disturbances, sleep bruxing, and reflux symptoms (Figure 13).
Young Adult 20–40
Pathologic tooth wear or significant relapse of orthodontic correction is usually addressed by fabrication of an occlusal orthosis. Continued grinding on the appliance is a hallmark for the bruxism triad. Self-reports of high stress may be due to repeated sleep disturbance and should not be disregarded. Evidence of GERD may be present on the teeth or maxillary palatal tissue. Bruxism may be acting as a protective reflex for the airway (Figure 14). There is a statistically significant association between childhood wear of the deciduous mandibular molars and canine teeth and the degree of whole-mouth wear observed 20 years later56 (Figure 15). For sleep bruxers with episodic sleep disturbances and reflux symptoms, this author has been using an anterior repositioning splint with success. The appliance is fabricated at < 40% of the patient’s maximum protrusion, minimizing the occlusal risk associated with advancement appliances (Figure 16 and Figure 17). The maxillary orthosis directs the patient’s closure into a protruded posture that opens the airway without being as bulky and restrictive as most MAAs.
Middle Adult 40–65
Bruxism will begin to wane as the risk of OSA increases. The impact of tooth grinding may be reduced but medical complications of OSA and GERD have increased. The effects of years of tooth and restoration damage may be apparent. Airway management becomes more difficult given the physical changes of middle age (Figure 18). As the literature elucidated, management of the airway during sleep improves all factors of the triad. CPAP is the gold standard for airway maintenance. For patients unwilling or unable to use CPAP, MAAs have the potential to diminish AHI, reduce sleep bruxism, and potentially lessen GERD (Figure 19 and Figure 20).
Mature 65 Older
The majority of patients in the mature group will have at least mild apnea. Medical issues related to overall health may be linked to OSA. Bruxism may be present but will be limited in its ability to clear the airway and reduce GERD. Patients may need to address tooth wear issues that have been compounded through years of neglect (Figure 21 and Figure 22). Comprehensive evaluation for signs and symptoms of OSA and referral for polysomnographic study is recommended. Patients with dentures should be encouraged to wear their dentures at night if sleep disruption is present (Figure 23 and Figure 24).
Conclusion
It has been demonstrated that a majority of dental patients present with tooth wear. However, sleep bruxers are a unique subset. Sleep bruxism is reflectively triggered by sleep microarousals. These disturbances in the sleep patterns are a natural occurrence but can also be caused by any disruption of airway patency or a significant reduction of esophageal pH. The bruxism triad is an attempt to explain the interlocking nature of bruxism, breathing, and erosion. These patients suffer a significant loss of tooth structure and restoration damage due to the increase in friction due to poor lubrication and roughened surfaces. Medical evaluation is key. CPAP, MAA, and GERD medications were proposed as possible treatment options for the bruxism triad patient.
References
1. De Leeuw R. Orofacial Pain Guidelines for Assessment, Diagnosis, and Management. 4th ed. Chicago, Ill: Quintessence; 2008.
2. Lavigne GJ, Montplaisir JY. Restless leg syndrome and sleep bruxism: Prevalence and associations among Canadians. Sleep. 1994;17:739-743.
3. Ohayon M, Li KK, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest. 2001;119:53-61.
4. Okeson JP, Kemper JT. In: Okeson JP. Management of Temporomandibular Disorders and Occlusion. 5th ed. St Louis, Mo: CV Mosby; 2003.
5. Ekfeldt A, Hugoson A, Bergendal T, Helkimo M. An individual tooth wear index and an analysis of factors correlated to incisal and occlusal wear in an adult Swedish population. Acta Odontol Scand. 1990;48:343-349.
6. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 2nd ed. American Academy of Sleep Medicine; 2005.
7. Lavigne GJ, Manzini C. Bruxism. In: Kryger M, Roth T, Dement W (eds). Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: W.B. Saunders Company; 2000:773-785.
8. Lavigne GJ, Kato T, Kolta A, Sessle BJ. Neurobiological mechanisms involved in sleep bruxism. Crit Rev Oral Biol Med. 2003;14:30-46.
9. Litonjua LA, Andreana S, Bush PJ, Cohen RE. Tooth wear: Attrition, erosion and abrasion. Quintessence Int. 2003;3:435-446.
10. Lavigne GJ, Manzini C, Kato T. Sleep Bruxism. In: Kryger M, Roth T, Dement W (eds). Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, Pa: W.B. Saunders Company; 2005:946-959.
11. Pintado MR, Anderson GC, DeLong R, Douglas WH. Variation in tooth wear in young adults over a two-year period. J Prosthet Dent. 1997;77:313-320.
12. Thie NMR, Kato T, Bader G, et al. The significance of saliva during sleep and the relevance of oromotor movements. Sleep Med Rev. 2002;6:213-227.
13. Lichter I, Muir RC. The pattern of swallowing during sleep. Electroencephalogr Clin Neurophysiol. 1975;38:427-432.
14. Moazzez R, Bartlett D, Anggiansah A. Dental erosion, gastro-oesophageal reflux disease and saliva: how are they related? J Dent. 2004;32:489-494.
15. Gibbs CH, Mahan PE, Mauderli A, et al. Limits of human bite strength. J Prosthet Dent. 1986;56:226-229.
16. Clark NG, Townsend GC, Carey SE. Bruxing patterns in man during sleep. J Oral Rehabil. 1984;11;123-127.
17. Nishigawa K, Bando E, Nakano M. Quantitative study of bite force during sleep associated bruxism. J Oral Rehabil. 2001;28:485-491.
18. Lavigne GJ, Cistulli PA, Smith MT (eds). Sleep Medicine for Dentists: A Practical Overview. Chicago, Ill: Quintessence Publishing Co, Inc; 2009.
19. Khoury S, Rouleau GA, Rompré PH, et al. A significant increase in breathing amplitude precedes sleep bruxism. Chest. 2008;134:332-337.
20. Baba K, Clark GT, Watanabe T, Ohyama T. Bruxism force detection by a piezoelectric film-based recording device in sleeping humans. J Oral Pain. 2003;17:58-64.
21. Okeson JP, Phillips BA, Berry DT, et al. Nocturnal bruxing events in subjects with sleep-disordered breathing and control subjects. J Craniomandib Disord. 1991;5:258-264.
22. Gold AR, Dipalo F, Gold MS, O’Hearn D. The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest. 2003;123:87-95.
23. Guilleminault C, Stoohs R, Clerk A, et al. A cause of excessive daytime sleepiness: the upper airway resistance syndrome. Chest. 1993;1993;104:781-787.
24. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217-1239.
25. Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906-1914.
26. Redline S. Epidemiology of sleep-disordered breathing. Semin Respir Crit Care Med. 1998;19:113-122.
27. Lavigne GJ, Rompré PH, Montplaiser JY. Sleep bruxism: Validity of clinical research diagnostic criteria in a controlled polysomnographic study. J Dent Res. 1996;75:546-552.
28. Lavigne GJ, Manzini C. Bruxism. In: Kryger M, Roth T, Dement W (eds). Principles and Practice of Sleep Medicine. 4th ed. Philadelphia, Pa: W.B. Saunders Company; 2005:946-959.
29. van Selms MK, Lobbezoo F, Wicks DJ, et al. Craniomandibular pain, oral parafunctions, and psychological stress in a longitudinal case study. J Oral Rehabil. 2004;31:738-745.
30. Lavigne GJ, Guitard F, Rompré PH, Montplaisir JY. Variability in sleep bruxism activity over time. J Sleep Res. 2001;10:237-244.
31. Camparis CM, Formigoni G, Teixeira MJ, et al. Sleep bruxism and temporomandibular disorder: clinical and polysomnographic evaluation. Arch Oral Biol. 2006;51:721-728.
32. Kato T, Montplaisir JY, Guitard F, et al. Evidence that experimentally induced sleep bruxism is a consequence of transient arousal. J Dent Res. 2003;82:284-288.
33. Ohayon MM, Li KK, Guilleminault C. Risk factors for sleep bruxism in the general population. Chest. 2001;119:53-61.
34. Bader GG, Kampe T, Tagdae T, et al. Descriptive physiological data on a sleep bruxism population. Sleep. 1997;20:982-990.
35. Oksenberg A, Arons E. Sleep bruxism related to obstructive sleep apnea: the effect of continuous positive airway pressure. Sleep Med. 2002;3:513-515.
36. Kobayashi Y, Shiga H. The relationship between sleep apnea, bruxism, and the time it took the patient to seek for treatment in TMD patients. J Oral Rehabil. 2002;29:885.
37. Yoshida K. A polysomnographic study on masticatory and tongue muscle activity during obstructive and central sleep apnea. J Oral Rehabil. 1998;25:603-609.
38. Sjöholm TT, Lowe AA, Miyamoto K, et al. Sleep bruxism in patients with sleep-disordered breathing. Arch Oral Biol. 2000;45:889-896.
39. The Gallup Organization: A Gallup survey on heartburn across America. Princeton, NJ: Gallup; 1988.
40. Fass R, Dickman R. Clinical consequences of silent gastroesophageal reflux disease. Curr Gastroanterol Rep. 2006;8:194-200.
41. Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. JAMA. 2002;287:1972-1981.
42. Lazarchik DA, Frazier KB. Dental erosion and acid reflux disease: an overview. Gen Dent. 2009;57:151-156.
43. Campisi G, lo Russo L, Di Liberto C, et al. Saliva variations in gastro-esophageal reflux disease. J Dent. 2008;36:268-271.
44. Landry ML, Rompré PH, Manzini C, et al. Reduction of sleep bruxism using a mandibular advancement device: an experimental controlled study. Int J Prosthodont. 2006;19:549-556.
45. Schönbeck AL, de Grandmont P, Rompré PH, Lavigne GJ. Effect of an adjustable mandibular advancement appliance on sleep bruxism: a crossover sleep laboratory study. Int J Prosthodont. 2009;22:251-259.
46. Gagnon Y, Mayer P, Morisson F, et al. Aggravation of respiratory disturbances by the use of an occlusal splint in apneic patients: a pilot study. Int J Prosthodont. 2004;17:447-453.
47. Arisaka H, Sakuraba S, Tamaki K, et al. Effects of wearing complete dentures during sleep on the apnea-hypopnea index. Int J Prosthodont. 2009;22:173-177.
48. Guda N, Partington S, Vakil N. Symptomatic gastro-oesophageal reflux, arousals and sleep quality in patients undergoing polysomnography for possible obstructive sleep apnea. Aliment Pharmacol Ther. 2004;20:1153-1159.
49. Demeter P, Visy KV, Magyar P. Correlation between severity of endoscopic findings and apnea-hypopnea index in patients with gastroesophageal reflux disease and obstructive sleep apnea. World J Gastroenterol. 2005;11:839-841.
50. Miyawaki S, Lavigne GJ, Mayer P, et al. Association between sleep bruxism, swallowing-related laryngeal movement, and sleep positions. Sleep. 2003;26:461-465.
51. Ing AJ, Ngu MC, Breslin AB. Obstructive sleep apnea and gastroesophageal reflux. Am J Med. 2000;108:120S-125S.
52. Tawk M, Goodrich S, Kinasewitz G, Orr W. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130:1003-1008.
53. Dayyat E, Kheirandish-Gozal L, San Capdevila O, et al. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136:137-144.
54. Noronha AC, de Bruin VM, Nombre e Souza MA, et al. Gastroesophageal reflux and obstructive sleep apnea in childhood. Int J Pediatr Otohinolaryngol. 2009;73:383-389.
55. Zettergren-Wijk L, Forsberg CM, Linder-Aronson S. Changes in dentofacial morphology after adeno-tonsillectomy in young children with obstructive sleep apnoea—a 5-year follow-up study. Eur J Orthod. 2006;28:319-326.
56. Knight DJ, Leroux BG, Zhu C, et al. A longitudinal study of tooth wear in orthodontically treated patients. Am J Orthod Dentofacial Orthop. 1997;112:194-202.